Currently, the pharmacotherapies approved for the treatment and prevention of osteoporosis fall under two categories: antiresorptive and anabolic agents. The antiresorptive agents inhibit the action of bone cells that degrade bone, whereas the anabolic agents promote bone formation. Teriparitide is the only approved anabolic agent. There are a number of FDA- approved classes of antiresporptive agents including bisphosphonates, selective-estrogen receptor modulators (SERMS), receptor activator of nuclear factor kappa beta (RANK) ligand inhibitors, and estrogen. The bisphosphonates are the most commonly used agents, which include oral (alendronate, ibandronate, and risedronate) and parenteral (ibandronate and zoledronic acid) preparations.
All of these agents have been shown to reduce the risk of vertebral fracture. Some of these have been shown also to reduce the risk of nonvertebral fractures, in some cases including hip fracture. Large randomized, placebo-controlled studies suggest that there is a 30% to 70% reduction in the risk of vertebral fractures and a 16% to 25% reduction in the risk of nonvertebral fractures with pharmacologic treatment.1,2 A number of general recommendations can be made regarding pharmacologic therapy for osteoporosis:
-
Bisphosphonates are usually considered first-line treatment for osteoporosis, particularly because of their positive effects on vertebral, nonvertebral, and hip fractures.
-
Second-line options include denosumab, raloxifene, strontium ranelate (in countries where available), and teriparatide.
-
Teriparatide use is often confined to high-risk patients, those with fractures, and in those with glucocorticoid- induced osteoporosis. Risk of osteosarcoma limits administration to two years.
-
Largely in light of the Women’s Health Initiative (WHI) study results and the availability of many other efficacious compounds, estrogen is no longer considered first-line therapy.
-
Combination use of antiresorptive drugs is generally not recommended because there is no current evidence of additional antifracture benefits, and there may be an increased risk of side effects and the over-suppression of bone turnover.
Patients deemed appropriate candidates for a pharmacologic therapy include:
-
Men and women who have experienced a hip or spine fracture
-
Those with osteoporotic range BMD determined by DXA
-
Persons with low bone mass (osteopenia) meeting a fracture risk (FRAX) threshold of more than 3% for hip and more than 20% for major osteoporotic fracture, based on the National Osteoporosis Foundation (NOF) recommendations3
-
Selected individuals without low bone mass but high fracture risk due to risk states such as use of glucocorticoids
Fracture risk thresholds may be different outside the United States.
Although a number of agents are available, the use of osteoporosis pharmacotherapy is also declining. Using commercial dispensing data, the use of oral bisphosphonates, the most commonly prescribed anti-osteoporosis pharmacotherapy, decreased by more than 50% from a peak of 31 million prescriptions dispensed in 2007 to only 14.7 million in 2012.4 Although less drastic, the sales of parenteral bisphosphonates for osteoporosis have also declined by 22% since its peak of 561,600 units in 2010 to 436,900 in 2012.4 It is speculated that declining treatment rates may be associated with a decrease in osteoporosis screening, lower physician initiation of therapy, and/or patient- or provider-initiated “drug holidays,” which are becoming more common because of perceived safety concerns of long-term bisphosphonate use.
The declining use of pharmacotherapy is also prevalent among those newly diagnosed with osteoporosis. In a recent study among women with incident osteoporosis, more than 64% of women had no indications of osteoporosis pharmacotherapy use one year after diagnosis.5 Pharmacotherapy use in those who have sustained a fracture is low. Recent evaluations have found that only 12% of Medicare beneficiaries had evidence of osteoporosis pharmacotherapy use six months after sustaining a fracture.6 In a managed care population, only 18% of fracture patients had indications for osteoporosis pharmacotherapy within 90 days of their fracture, and only 23% appeared to use osteoporosis pharmacotherapy within a year postfracture.7 Studies in hip fracture patients found that 19% to 24% received therapy within one year postfracture,8,9 and that pharmacotherapy use posthip fracture has declined from 40% in 2002 to 21% in 2011.9
- 1. Kanis JA1, Burlet N, Cooper C, et al.: European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 2008 Apr;19(4):399-428. doi: 10.1007/s00198-008-0560-z. Epub 2008 Feb 12.
- 2. Sambrook P1, Cooper C: Osteoporosis. Lancet 2006 Jun 17;367(9527):2010-2018.
- 3. Cosman F, deBeur SJ, LeBoff MS, et al.: Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 2014;25(10):2359–2381. doi: 10.1007/s00198-014-2794-2.
- 4. a. b. Wysowski DK, Greene P.: Trends in osteoporosis treatment with oral and intravenous bisphosphonates in the United States, 2002–2012. Bone 2013;57(2):423-428.
- 5. Siris ES, Modi A, Tang J, et al.: Substantial under-treatment among women diagnosed with osteoporosis in a US managed-care population: A retrospective analysis. Curr Med Res Opin 2014 Jan;30(1):123-130. doi: 10.1185/03007995.2013.851074. Epub 2013 Oct 25.
- 6. Liu SK, Munson JC, Bell JE, et al.: Quality of osteoporosis care of older Medicare recipients with fragility fractures: 2006 to 2010. J Am Geriatr Soc 2013 Nov;61(11):1855-1862. doi: 10.1111/jgs.12507. Epub 2013 Oct 28.
- 7. Wilk A, Sajjan S, Modi A, et al.: Post-fracture pharmacotherapy for women with osteoporotic fracture: Analysis of a managed care population in the USA. Osteoporos Int 2014 Dec;25(12):2777-2786. doi: 10.1007/s00198-014-2827-x. Epub 2014 Aug 12.
- 8. Antonelli M, Einstadter D, Magrey M.: Screening and treatment of osteoporosis after hip fracture: Comparison of sex and race. J Clinic Densitometry 2014;17(4):479-483. doi: 10.1016/j.jocd.2014.01.009. PMID: 24657109.
- 9. a. b. Solomon DH, Johnston SS, Boytsov NN, et al.: Osteoporosis medication use after hip fracture in US patients between 2002 and 2011. J Bone Miner Res 2014 Sep; 29(9):1929-1937. Published online 2014 Aug 20. doi: 10.1002/jbmr.2202 PMCID: PMC4258070.
Edition:
- 2014

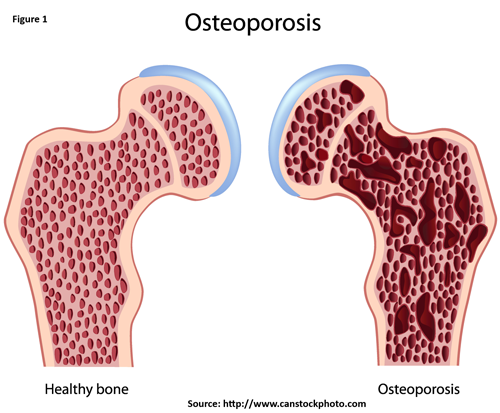
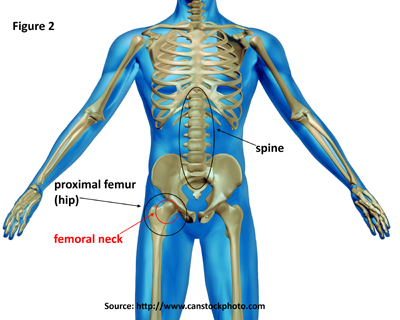
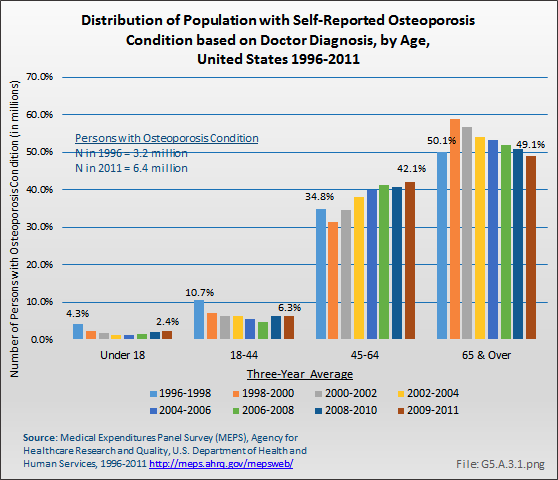
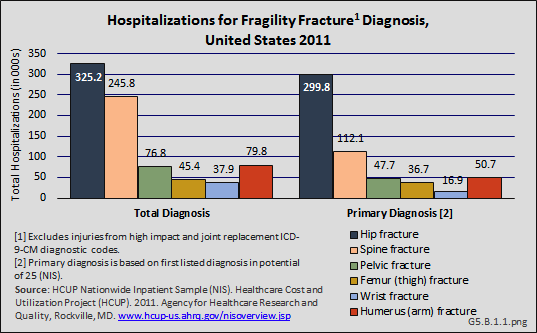
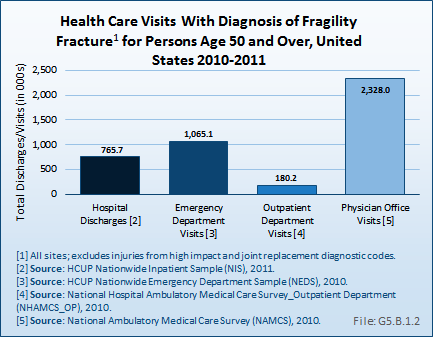
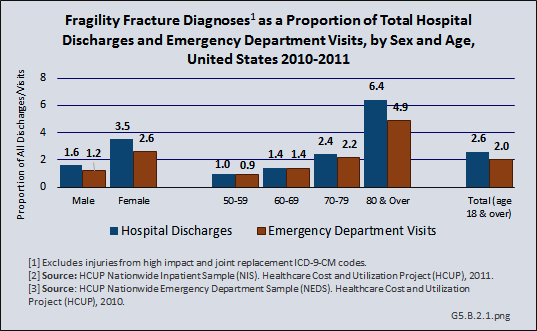
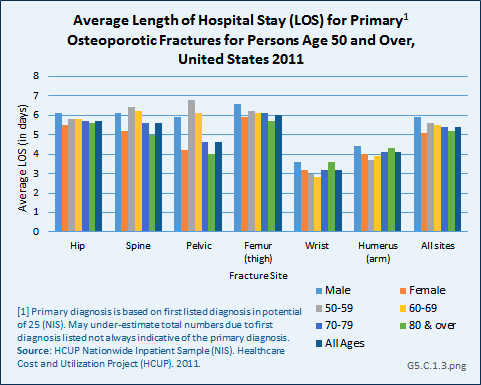
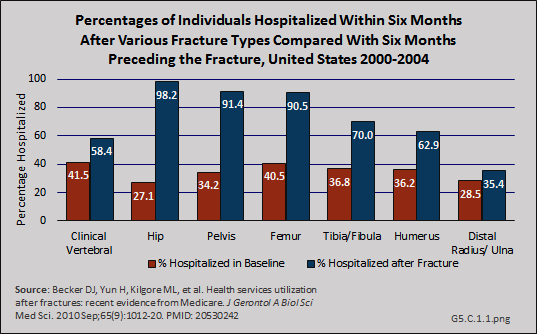
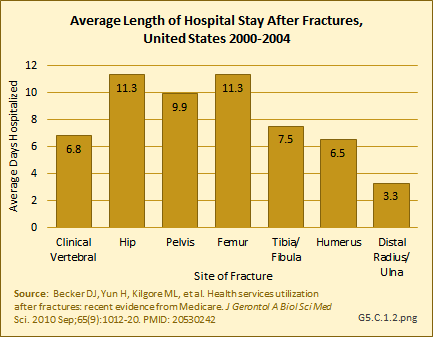
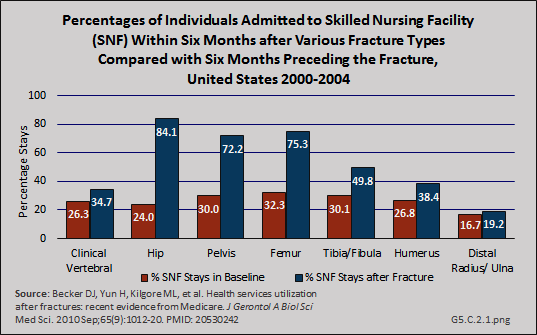
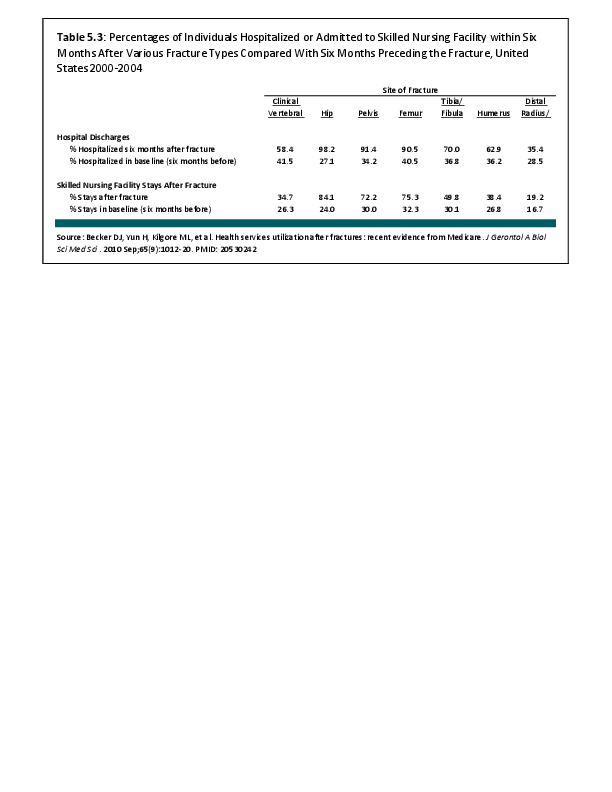
 Download as CSV
Download as CSV